Capture & Identify unique molecular fingeprints
The Open-SourceRaman Spectrometer
Raman spectroscopy lets you precisely analyze the chemical composition of substances without damaging them. On this site, you’ll find everything you need to build your own Raman spectrometer using off-the-shelf components – including parts lists, detailed build instructions, calibration tips, and software.
Made for Makers
Open-Source
Under 1000$
Quick Introduction
About Raman Spectroscopy
A powerful analytical tool
Raman spectroscopy is a powerful technique for the qualitative and quantitative analysis of chemical substances.
A sample is briefly irradiated with a laser, and the scattered light is collected, filtered, and detected by a sensor – resulting in a characteristic spectrum.
Common Applications & Capabilities
What it can analyze
SubstanceIdentification
- Pharmaceuticals
- Drugs of abuse
- Organic compounds
Chemicals &Compounds
- Solvents
- Reagents
- Acids
MaterialScience
- Plastics
- Polymers
- Semiconductors
BiologicalSamples
- Tissues
- Microorganisms
- Cultures
Non-destructive
Liquid, solid & gaseous
In-situ monitoring
Scattering Photons
The Raman Effect
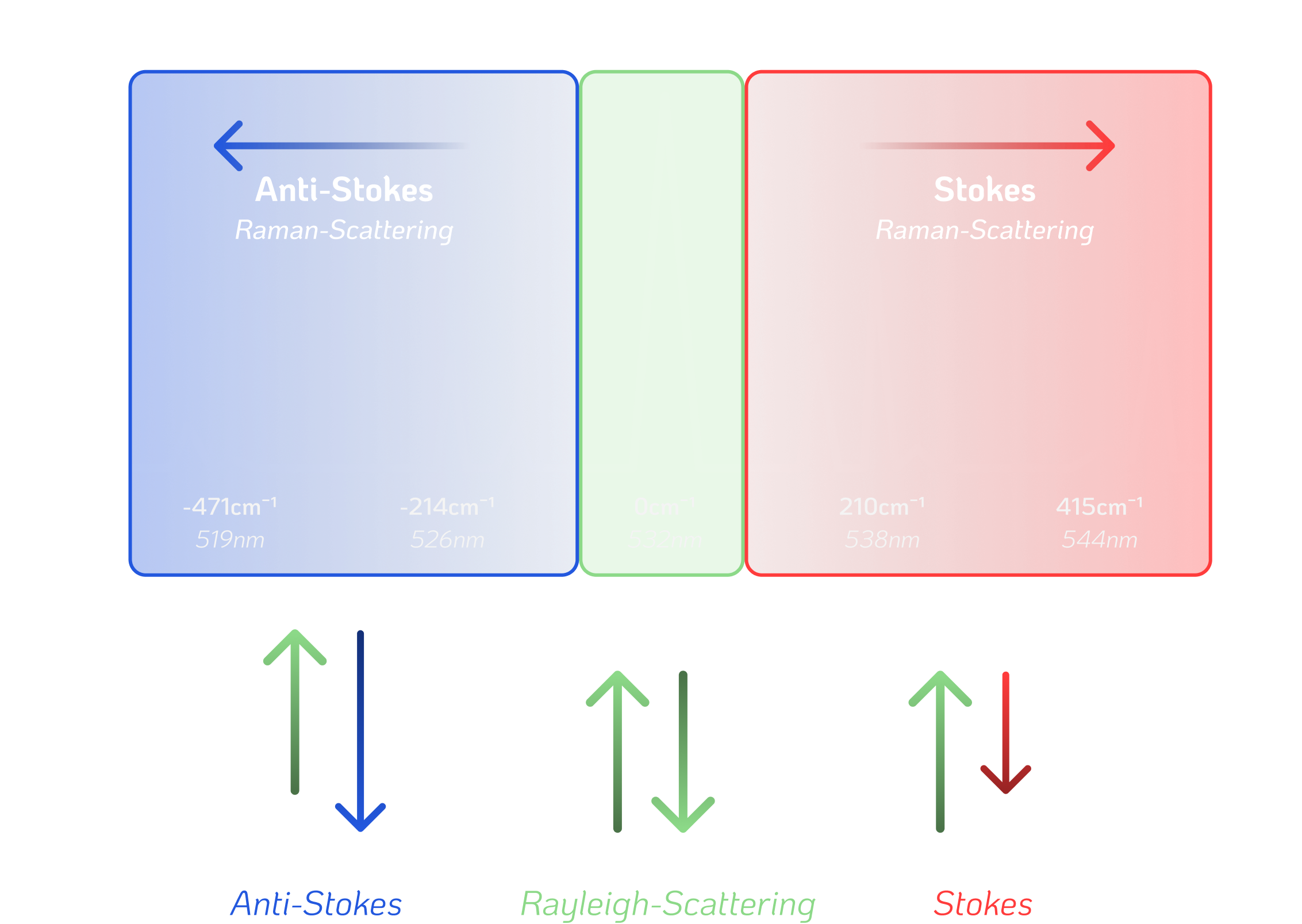
In simple terms, a Raman spectrometer uses a laser to focus light onto a small spot on a sample. The photons excite molecular vibrations that scatter the light and uniquely shift its wavelength – known as Raman scattering. By filtering out the Rayleigh-scattered, original laser light, we isolate and capture the desired signal, guide it into a spectrometer and plot it as a unique spectrum.

Rayleigh Scattering
- so-called inelastic scattering
- affects about 1 in 10 million photons
- incident light changes direction and energy
- the molecule gains or loses energy = wavelength shift

Raman Scattering
- so-called elastic scattering
- the most common, visible everyday scattering
- incident light only changes direction
- wavelength stays the same

Fluorescence
- e.g., the glow under UV
- incident light is absorbed and re-emitted
- much stronger than the Raman signal
- emitted in random directions
The Output
First Spectrum
Raw spectrum of paracetamol as it’s received, without any subtraction of the baseline. The red one is the reference, which is already post-processed with baseline-correction, smoothing and peak-fitting.
Broad Coverage
The Stokes-shift 600-3000 cm^-1 captures the fingerprint region and more.
Precise Identification
Easily allows for sufficient resolution to detect substances.
Safe Operation
While less hazardous, appropriate laser safety goggles must be worn at all times!
In 9 rough steps
From Emission to Spectrum
Step 1
Laser Emission - 532nm
A narrow laser beam of known wavelength - in our case 532 nm - is emitted from a cheap laser pointer
Step 2
Infrared Reduction (optional)
The high-energy portion of Infrared - especially with cheap laser modules - is selectively reflected by a mirror at wavelengths above our laser, as it would otherwise oversaturate the spectrometer's sensor.
Step 3
Dichroic Beamsplitter - 550nm Cut-On
Our beam hits the dichroic mirror, which reflects any wavelength below 550nm and transmits anything higher. At 45° orientation, our incident beam is subsequently reflected into the microscope objective.
Step 4
Microscope Objective - 20x inf.
By passing through the objective, the collimated beam is focused onto a tight spot at the objective’s specified working distance. It concentrates the photons of our beam onto the sample and allows scattered light to pass inversely.
Step 5
Sample
The inert quartz glass cuvette, is loaded with either a solid or liquid substance. Our laser light excites the molecule, causing common scattering and an extremely tiny fraction of Raman scattering to occur. Some of it is collected back by and through the objective.
Step 6
Signal Separation
Scattered light coming from our sample is again separated by the dichroic mirror, leaving almost all of our initial wavelength to be reflected and the stokes portion - Raman-shifted signal higher than our excitation laser wavelength - transmitted.
Step 7
Additional Clean-Up - Longpass Filter Cut-On 550nm
Since the ‘normal’ scattering is so intense, and the Raman effect so weak, the signal needs to be filtered again to reduce noise on the sensor. So, again, anything below 550nm is not allowed to pass and we end up with practically just the desired Raman-shifted wavelengths.
Step 8
Final Focusing
Our clean parallel Raman beam still needs to be focused into our spectrometer unit - optionally via optical fiber - to maximize signal quality. This is done by a simple lens setup and a linear stage to precisely adjust the focus.
Step 9
Data Collection - Spectrometer
If everything is aligned properly, the signal is captured with an exposure of a couple seconds and may then be analysed. Using various algorithms, the spectrum is cleaned up to increase legibility and its characteristics. From that our substance can be identified - and much more.
incl. Tax & Shipping
Bill of Materials
Spectrometer
B&W Tek BTC-110S - Ebay (used)
Laser 532nm
30mW - Aliexpress
Longpass Filter 550nm
FELH0550 - Thorlabs
Dichroic Mirror 550nm
DMLP550 - Thorlabs
Bandpass Filter 532nm
532nm x 10nm (#65640) - Edmund Optics
Kinematic Mounts 2pc.
KM100 - Thorlabs
Microscope Objective 20x ∞
Any infinity focused - Ebay (used)
Quartz Cuvette
Any, cuvette glass width > objective WD - Ebay (used)
Fiber Optic Cable
Any, SMA905-SMA905 1m VIS-IR 200um - Aliexpress
3D-Printing Filament
Any, high light absorption PETG-CF (Black) - Amazon
$985
Total Cost
Software
Alpha versions of the acquisition and post-processing software are available now and allow serial communication with the B&W Tek spectrometer.
Expectations & Notes
About this Project
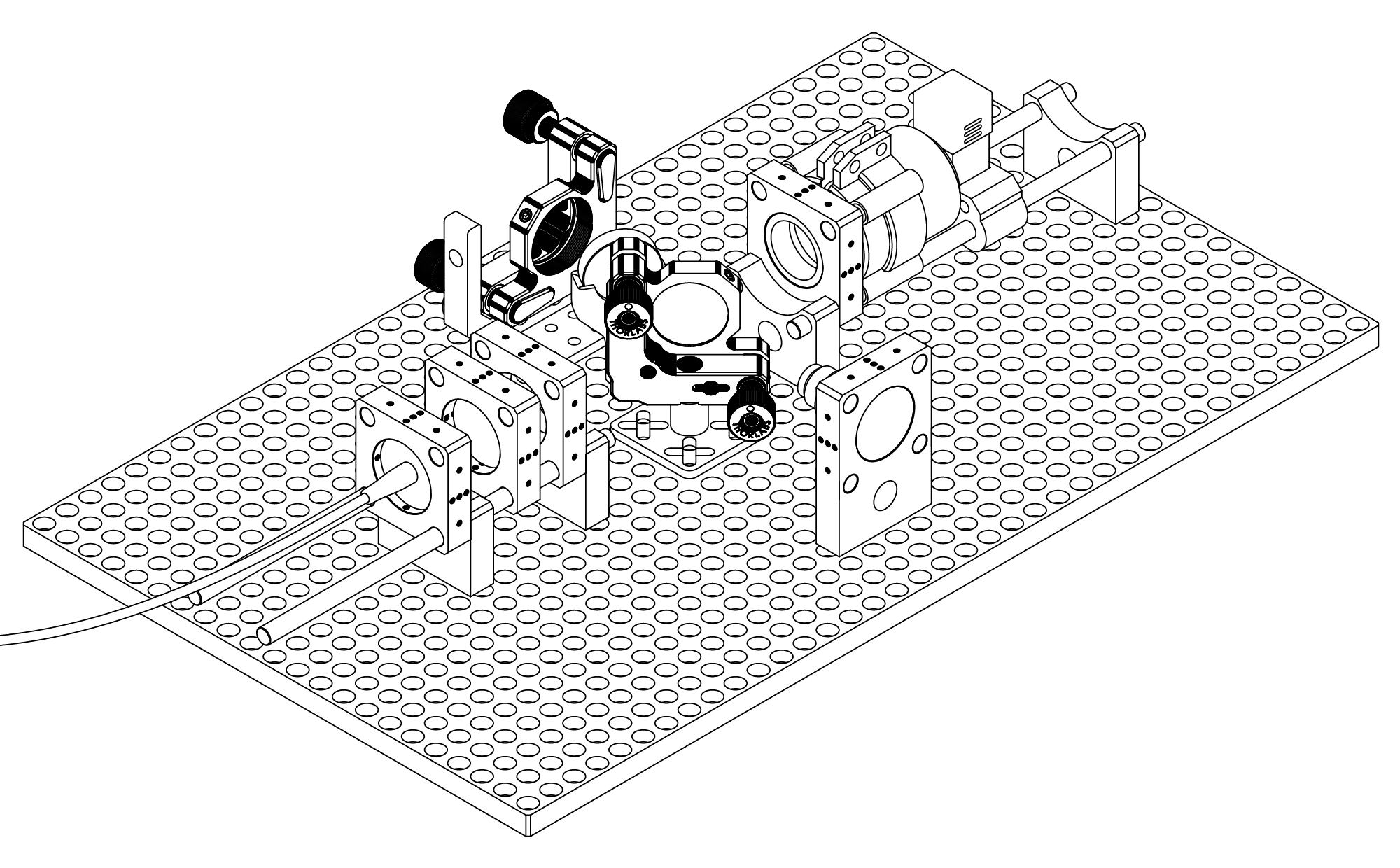
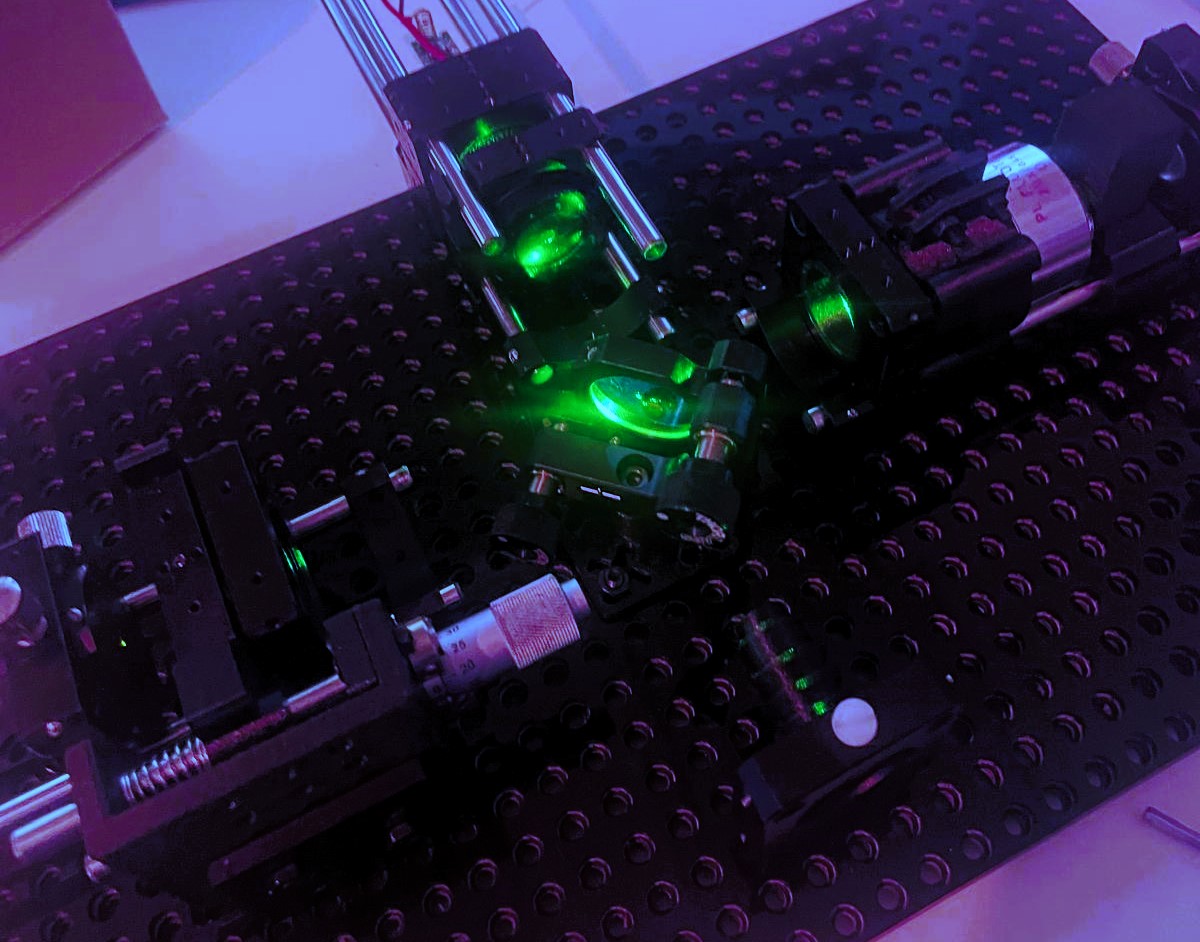
This project is a documentation of my journey and is meant to serve as a beginner-friendly compilation for anyone wanting to get into Raman Spectroscopy – and hopefully end up with a working prototype for under or around 1000$. I am by no means an optical engineer or physicist, so take any statement, that isn’t annotated with a direct citation of a source, with a grain of salt. I’ll do my best to incorporate the source material, whenever an important consideration warrants it.
Feel free to get in touch with me regarding any feedback, possible improvements or criticism – it is greatly appreciated and very much encouraged, as it contributes to an exhaustive, thorough standard, I’d love for this project to achieve.
FAQ
Frequently Asked Questions
In case any question remains unanswered, feel free to contact me and I’ll try my best to respond.
Building one requires some experience with 3d-printing, electronics, software and ideally optics. The hardware setup is mechanically simple but optical alignment and signal optimization take patience. It’s a great learning project but not plug-and-play.
Mostly solids and powders. Transparent liquids also work. But highly fluorescent or dark samples (e.g. plant matter, some dyes) can overwhelm the Raman signal. Some materials may require special preparation or extraction.
Typical exposure times range from 1 to 10 seconds, depending on your sample and laser power. Weak signals may need longer integration or many exposures.
No, this setup can only provide you with qualitative results – meaning the most present Raman-active substances. For quantitative measurements you would need to use some certified calibration samples and use a detector with higher resolution. In general, I’d advise against using it for that purpose.
If you get the same spectrometer unit off of ebay, you can use the notably very old SpectrumStudio software to capture spectra and communicate with the spectrometer. I also provide the software I wrote in Python, which works essentially the same, but is kept to a minimum of functionality – some of which is not provided in the original software. The post-processing of the spectra can be done in a variety of programs. Though I’m also working on implementing that directly into the software, even if you own a different spectrometer and choose to just use it to easily process your exported data.
Websites & Publications
Further Reading
- Raman lasers are powerful enough to cause permanent eye damage in milliseconds – even and especially from reflections!
- Always wear proper laser safety goggles rated for your laser’s wavelength! This project deals with visible 532 nm, opposed to invisible and more difficult to handle infrared light.
- Cheap green laser diodes oftentimes still emit a very powerful fraction of infrared light, if no adequate filter is used – they should not be able to light dark paper on fire without a lens.
- Keep your laser beam enclosed whenever possible to avoid accidental exposure and mitigate stray light as much as possible – this is also important for a good signal-to-noise ratio.
- Analogous to working with ionizing radiation, ALARA “as low as reasonably achievable” is a good sentiment here, meaning run the laser diode at the lowest working power to achieve sufficient signal – cheap laser diodes often flicker or perform badly at very low running current. Trial and eror is the most reliable approach here.
- Follow your local laser safety regulations and ideally take some laser safety classes – using a lower power laser also reduces risks of damage proportionally, so following your local laws can be eyesight-protecting.
- Handling of hazardous substances and chemicals can pose significant health hazards, always read the safety data sheet (SDS) before handling – the warning labels are there for a reason and reading data sheets is surprisingly interesting.
- Most solvents and their vapors are highly flammable and don’t pair well with unisolated, open electricity.
- Use appropriate PPE when working with samples – latex gloves are used at all times when dealing with optical components and since you will be wearing your laser goggles anyway, you might only need a respirator when dealing with unknown samples of potentially hazardous chemicals.
- Optical components are incredibly fragile – and expensive – and should only be exposed to a clean environment whenever needed. Never touch them with your bare hands, don’t blow on them, minimize dust exposure with a glovebox – more on this in the instructions.
- Don’t rely exclusively on this writeup!
- This is NOT a certified analytical tool and its results should be treated as such! For (qualitative) educational and exploratory use only.























